High Hemoglobin F in a Saudi Child Presenting with Pancytopenia
Abdullah A Elhosiny*, Alaa E Kintab, Soheir S Adam
DOI10.21767/2471-805X.100002
Abdullah A Elhosiny1*, Alaa E Kintab2 and Soheir S Adam3
School of Medicine, King Abdul-Aziz University, Jeddah, Saudi Arabia
Department of Pediatrics, King Abdul- Aziz University Hospital, Jeddah, Saudi Arabia
Department of Hematology, King Abdul- Aziz University Hospital, Jeddah, Saudi Arabia
- *Corresponding Author:
- Abdullah A Elhosiny
King Abdul-Aziz University, Jeddah, KSA
Tel: +966 566 625 736
E-mail: ab.alhusayni@gmail.com
Received date: September 12, 2015 Accepted date: November 06, 2015 Published date: November 16, 2015
Citation: Elhosiny AA. High Hemoglobin F in a Saudi Child Presenting with Pancytopenia. J Pediatr Care. 2015, 1:1. doi:10.21767/2471-805X.100002
Abstract
Saudi Arabia has a high prevalence of sickle cell disease with a wide spectrum of clinical presentations, and sickle cell disease patients are divided according to the clinical phenotypes. Sickle cell disease patients who have higher expression of hemoglobin F level are reported to experience milder disease since high hemoglobin F is thought to ameliorate the disease manifestations. However, the effects are not consistent since hemoglobin F is not equally distributed among erythrocytes. Multiple factors can help determine the level of fetal hemoglobin in adults. Here we report a case of a Saudi patient who presented with her first crisis at the age of 6 years.
Keywords
Sickle Cell Disease; High Hemoglobin F
Abbreviation
AI: Arab-Indian; G6PD: Glucose 6 Phosphate Dehydrogenase; Hb: Hemoglobin; HbF: Hemoglobin F; HbS: Hemoglobin S; HPFH: Hereditary Persistence of Fetal Hemoglobin; QTL: Quantitative Trait Loci; SCA: Sickle Cell Anemia; SCD: Sickle Cell Disease; SNP: Single Nucleotide Polymorphism.
Introduction
Hemoglobin F (HbF) molecule is composed of 2α globin polypeptide chains and 2γ polypeptide chains. The γ globin chains are encoded by 2 nearly identical genes (HBG1 and HBG2), which are located within the β globin-gene-cluster on chromosome 11 [1].
HbF protects against many of the clinical complications of sickle cell disease (SCD), because of its ability to hinder the polymerization of deoxygenated hemoglobin S (HbS) [2,3].
Persistence of HbF expression is heritable, it can be influenced by 2 known major quantitative trait loci (QTL), which are (HBS1LMYB intergenic region and BCL11A), and other factors associated with the β globin-gene-cluster [1].
Hereditary persistence of fetal hemoglobin (HPFH), is a group of disorders which are classified into deletion and non-deletion subtypes. The deletion subtype is caused by a large deletion in the β globin-gene-cluster leading to a pan-cellular distribution of HbF and a clinically normal phenotype, whereas the non-deletion subtype is caused by point mutations in the β globin-gene-cluster leading to a hetero-cellular distribution of HbF.
Different haplotypes with SCD have considerable variation in HbF level, suggesting different QTL that modifies HbF genes (HBG1 and HBG2) expression, or other genetic factors that have an effect on them.
Some single nucleotide polymorphisms (SNPs) have been identified in these two QTL, and it explained 5%-40% of HbF variation among different SCD haplotypes [1]. However, other factors that contribute to the variation remain unidentified.
A previous study found that the Saudi or Arab-Indian (AI) β globingene- cluster haplotype found mainly in Saudi Arabia had the highest HbF level with different distribution. Thus SCD patients who carry this haplotype present with a milder phenotype [1].
This is a case of SCD with high HbF presenting with an aplastic crisis.
Case Report
A 6 years old, previously well Saudi girl presented to the Emergency Department at King Abdul-Aziz university hospital on 8th of April 2015 complaining of drowsiness, high-grade fever and cough for 2 days. Her drowsiness became progressive, not improving, associated with weakness, continuous fever, and loss of appetite. Her mother reported that she became pale and jaundiced with a change in urine and stool color into dark. She lives with her parents, who are consanguineous as well as her healthy younger siblings. She had no history of recent travel and her family history is negative for chronic diseases and hemoglobinopathies including sickle cell disease (SCD) and thalassemia. She was a healthy child with no history of previous illness or hospital admissions.
On examination, there were crepitations all over the chest with transmitted sound. Physical examination was otherwise unremarkable.
The Initial blood assessment showed a low hemoglobin (Hb) level of 5.0 g/dL (10.2 - 15.2) and thrombocytopenia of 97 K/uL (150 – 450). She received one pack of red blood cells and was then admitted to the hospital for further investigation.
The initial impression was:
1. Chest infection: viral or bacterial pneumonia.
2. Anemia possibly secondary to hematinics deficiency, hemoglobinopathy, glucose 6 phosphate dehydrogenase (G6PD) deficiency, or malaria.
She stayed in the hospital for almost 1 week. During her admission, she was complaining of a productive cough, epigastric pain with vomiting episodes. She was started on cefuroxime for community-acquired pneumonia and proton pump inhibitor for epigastric pain.
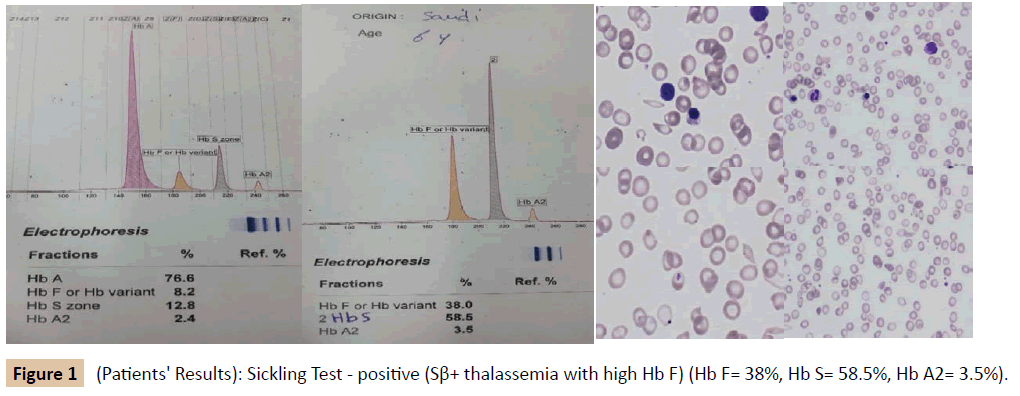
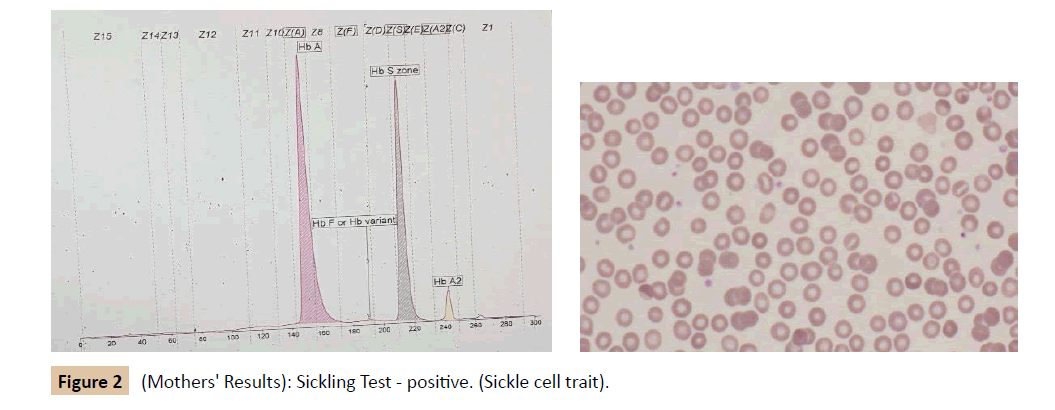
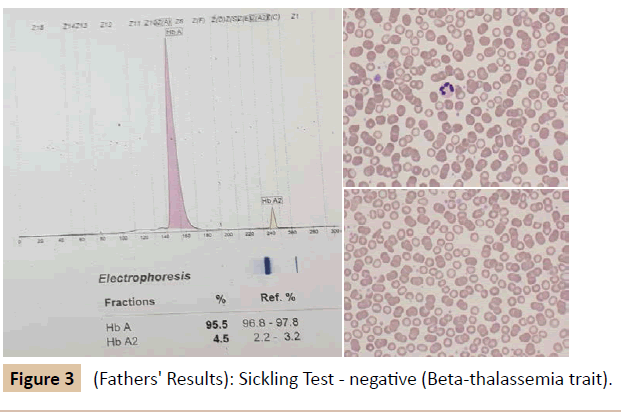
During her stay in the hospital, a full investigation of the cause of anemia was performed (Table 1 and Figure 1). Based on the results, she was diagnosed as SCD (Sβ+ Thalassemia) presenting with an aplastic crisis. A hematological referral was sent and it was recommended to start the patient on penicillin prophylaxis, folic acid, and vitamin D3 drops. Later on, she received pneumococcal, meningococcal and hemophilus influenza vaccines, and she was booked for an echocardiogram and abdominal ultrasound. A family study was performed (Tables 2, 3, Figures 2 and 3). The patient improved and was discharged from our hospital on 13th of April 2015 with a follow-up appointment in the clinic.
| Lab results upon admission 08/04/2015 | Results | Unit | Reference range | |||||
|---|---|---|---|---|---|---|---|---|
| Red Blood Cell Count (RBC) | 2.19 | M/uL | 3.9 – 5.2 | |||||
| White Blood Cell Count (WBC) | 8.19 | K/uL | 5.0 - 17.0 | |||||
| Hematocrit (HCT) | 14.7 | % | 34 - 48 | |||||
| Mean Cell Hemoglobin (MCH) | 22.8 | pg | 32 - 36 | |||||
| Mean Cell Volume (MCV) | 67.1 | FL | 78 - 94 | |||||
| Reticulocytes count | 2.05 | % | 0.5 – 1.5 | |||||
| Platelet Count (PLT) | Unit: K/uL | Reference Range: 150 – 450 | ||||||
| 29/04/2015 | 10/04/2015 | 08/04/2015 | 08/04/2015 | 08/04/2015 | 08/04/2015 | |||
| 297 | 118 | 99 | 101 | 84 | 97 | |||
| Hemoglobin (Hb) | Unit: g/dL | Reference Range: 10.2 - 15.2 | ||||||
| 29/04/2015 | 10/04/2015 | 08/04/2015 | 08/04/2015 | 08/04/2015 | 08/04/2015 | |||
| 10.0 | 9.2 | 9.1 | 4.8 | 4.9 | 5.0 | |||
| Test done | Results | |||||||
| Blood and urine cultures | Negative | |||||||
| Parvo Virus B19 IgM | Negative | |||||||
| Parvo Virus B19 IgG | Positive | |||||||
| EBV | Negative | |||||||
| CMV | Negative | |||||||
| HIV | Negative | |||||||
| Hepatitis | Negative | |||||||
| Malaria | Negative | |||||||
Table 1: Patients' laboratory results.
| Lab results | Results | Unit | Reference range |
|---|---|---|---|
| Red Blood Cell Count (RBC) | 4.92 | M/uL | 3.9 – 5.2 |
| Hemoglobin (Hb) | 13.8 | g/dL | 10.2 - 15.2 |
| Hematocrit (HCT) | 40.8 | % | 34 - 48 |
| Mean Cell Hemoglobin (MCH) | 28.0 | pg | 32 - 36 |
| Mean Cell Volume (MCV) | 82.9 | FL | 78 - 94 |
| Platelet Count (PLT) | 245 | K/uL | 150 – 450 |
Table 2: Mothers' laboratory results.
| Lab results | Results | Unit | Reference range |
|---|---|---|---|
| Red Blood Cell Count (RBC) | 6.1 | M/uL | 4.4 - 5.8 |
| Hemoglobin (Hb) | 13.6 | g/dL | 10.2 - 15.2 |
| Hematocrit (HCT) | 43.2 | % | 34 - 48 |
| Mean Cell Hemoglobin (MCH) | 22.2 | pg | 32 - 36 |
| Mean Cell Volume (MCV) | 70.6 | FL | 78 - 94 |
| Platelet Count (PLT) | 353 | K/uL | 150 – 450 |
Table 3: Fathers' laboratory results.
Discussion
The persistence of HbF in high levels beyond the neonatal life is genetically determined. In SCD patients, high levels of HbF were found to be protective. The level of HbF in Saudi haplotype or Arab-Indian (AI) haplotype of SCD is 3-4 folds higher than that in the African haplotype [3]. Comparison of the levels of HbF in many different haplotypes including the regions; Africa, the Middle East, and the Indian subcontinent, showed the Senegal and the Saudi haplotypes had the highest levels of HbF compared to the Bantu and Benin haplotypes, which had the lowest levels [1]. The Senegal and the Saudi-Indian haplotypes were found to share a similar single nucleotide polymorphism (SNP) (rs7482144), which other haplotypes like Bantu and Benin lacked [1]. This SNP is thought to have a strong effect on the expression of HBG2 gene, thus increasing the levels of HbF. However, although the Senegal and the Saudi haplotypes shared this SNP, the Saudi still had higher levels of HbF compared to Senegal. They also found that SCD patients of Saudi descent with typical African-derived haplotype still had higher levels of HbF compared with non-Saudi patients of African descent. These observations suggest that there are perhaps environmental or other genetic elements that could be linked to these haplotypes that affect HbF genes (HBG1and HBG2) transcription, thus increasing the levels of HbF [1]. A large study conducted in Saudi Arabia concluded that certain regulatory elements that are yet to be discovered, modulate the effects of the known cis- and trans-acting regulators may be responsible for the higher levels of HbF in the AI haplotype [3].
In another study conducted to compare the levels of HbF between AI haplotype with homozygous HbS and the AI haplotype with HbS-β thalassemia, found that both groups had similar HbF levels and approximately similar distribution. However, they could not detect any association of these SNPs with HBG2 gene expression, perhaps due to the small sample size studied [2].
Occasionally, a mutation can occur in HbF genes (HBG1 and HBG2) or in the SNPs that modulate their expression, leading to what is known as hereditary persistence of fetal hemoglobin (HPFH.) [1].
Future studies should focus on identifying factors that contribute in increasing the levels of HbF in the Saudi haplotype to shed light on this unique phenotype and improve the individualized care of this patient population.
References
- Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, et al. (2011) Fetal hemoglobin in sickle cell anemia. Blood 118: 19-27.
- Alsultan A, Ngo D, Bae H, Sebastiani P, Baldwin CT, et al. (2013) Genetic studies of fetal hemoglobin in the Arab-Indian haplotype sickle cell-beta (0) thalassemia. American journal of hematology 88: 531-2.
- Ngo D, Bae H, Steinberg MH, Sebastiani P, Solovieff N, et al. (2013) Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood cells, molecules & diseases 51: 22-6.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences