Comparing the Effects of Zinc Supplementation as Adjunct to the Conventional Therapy and Placebo on Morbidity in Children with Pneumonia Between Ages 1 Year to 5 Years
Arun Kumar Singh, Muhammad Ashraf Sultan
DOI10.21767/2471-805X.100032
Arun Kumar Singh1* and Muhammad Ashraf Sultan2
1Department of Pediatrics and Adolescents, B.P. Koirala Institutes of Medical Sciences, Dharan, Nepal
2Tropical Pediatrics (Liver Pool), King Edward Medical University, Mayo Hospital, Lahore, Pakistan
- *Corresponding Author:
- Dr. Arun Kumar Singh
Paedtrician and Neonatalogist, Department of Pediatrics and Adolescents
B.P. Koirala Institute of Health Sciences, Dharan, Nepal
Tel: 009779844241397
E-mail: arunsinghnepal@gmail.com
Received Date: May 23, 2017; Accepted Date: May 26, 2017; Published Date: May 31, 2017
Citation: Singh AK, Sultan MA. Comparing the Effect of Zinc Supplementation as Adjunct to the Conventional Therapy and Placebo on Morbidity in Children with Pneumonia between Ages 1 Year to 5 Years. J Pediatr Care 2017, 3:3 DOI:10.21767/2471-805X.100032
Abstract
Background: Pneumonia is one of the leading causes of morbidity and mortality in children younger than 5 years of age. Treatments are available for timely management of pneumonia but mortality is still high in developing countries like Pakistan. Zinc may have an important protective role in cases of childhood pneumonia and can help in reducing potential complications of pneumonia and can also help to reduce the incidence of mortality in children less than five years of age. So we conducted this study to find the therapeutic role of zinc as an adjunct to standard therapy for pneumonia in comparison to placebo.
Objective: To compare the effect of zinc supplementation and placebo on morbidity in children with pneumonia age 1 year to 5 years in one year duration.
Study duration: One year.
Methodology: This randomized control trial was conducted in the Department of Pediatrics Unit-I, King Edward Medical University/Mayo Hospital, Lahore, Pakistan. After the consent was taken, 150 children from 1 year to 5 years of ages with pneumonia consistent with World Health Organization (WHO) acute respiratory infections (ARI) definition along with crepitation’s on auscultation were registered by non-probability purposive sampling and were randomized into treatment group (Group A) and placebo group (Group B). Seventy five children were supplemented with zinc for 15 days while 75 children were supplemented with placebo. Outcome measure was duration of hospital stay in both groups.
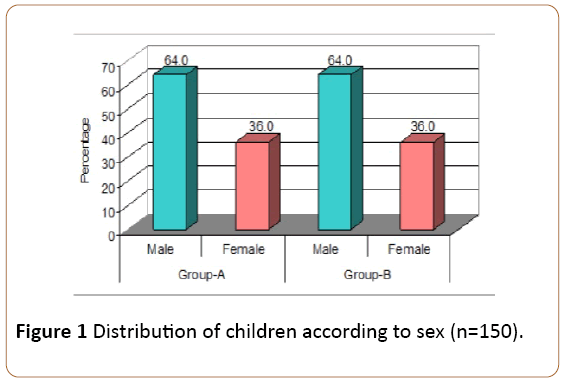
Results: The mean age of children in zinc therapy group was 3.01 years while the mean age of children in placebo group was 2.85 years. In zinc therapy group, there were 48 (64.0%) male and 27 (36.0%) female children. In placebo group, there were 48 (64.0%) male and 27 (36.0%) female children.
The mean hospital stay was 3 ± 1 days in zinc group while 7 ± 3 days in placebo groups (P<0.05) and showing that zinc therapy is better than placebo.
Conclusion: Zinc supplementation as adjunct to the conventional therapy for management of pneumonia reduces hospital stay.
https://bluecruiseturkey.co
https://bestbluecruises.com
https://marmarisboatcharter.com
https://bodrumboatcharter.com
https://fethiyeboatcharter.com
https://gocekboatcharter.com
https://ssplusyachting.com
Keywords
Children; Duration of hospital stay; Pneumonia; Zinc supplementation; Placebo; Mortality; Morbidity
Introduction
Pneumonia is the leading killer of children, causing an estimated 1.9 million deaths worldwide under the age of 5 years. About 90% of these deaths occur in the developing world [1].
Pneumonia is an inflammation of the parenchyma of the lungs. It is caused by bacteria, viruses and others microorganisms [2]. Among bacteria Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Mycobacterium tuberculosis, Atypical mycobacterium, Salmonella, Esherichia coli and Pnuemocystis jirovecii must be considered [3]. Other common causes include Respiratory Syncytial Virus, Parainfluenza viruses, Adenoviruses and Metapneumovirus [4]. Although most cases of pneumonia are caused by microorganisms, non-infectious causes include aspiration of food or gastric acid, foreign bodies, hydrocarbons and lipoid substances, hypersensitivity reactions and drugs or radiation induced pneumonitis [5,6].
In Pakistan acute respiratory illness is the leading cause of death in young children responsible for 20% to 30% of all children death under age five years [7,8].
Pakistan was fourth in ranking of countries by the total number of under-5 deaths, with an estimated 565,000 deaths in the year 2000 [2]. It is more common in low socio-economic status in those who live in crowded conditions and who have not been breast feed. Seasonal variation in Pakistan, as noted for other areas of globe is seen with larger peak in winter [9].
Pneumonia is a leading cause of morbidity and mortality in children less than 5 years is responsible for two million deaths each year in children under five year of age accounting for 19% of annual deaths in this age group [1].
Approximately 95% of the pneumonia related deaths occurs in developing countries, and the younger age and malnutrition have the highest risk of death [10].
Zinc deficiency is common in children in developing countries [5]. Zinc deficiency has been known since the 1960s [11]. Zinc may have an important protective role in the respiratory epithelium and deficiency may enhance airway inflammation and epithelial damage [12], impairs many cellular and humoral immune functions including lymphocyte number and function [13]. Animals had decreased inflammation and cellular damage in the presence of zinc [11]. In developing countries, oral zinc (20 mg) helps accelerate recovery from severe pneumonia [14].
Pneumonia although is the most common reason for seeking medical advice and is among one of the commonest cause of death in children in developing countries [15].
Zinc therapy has been reported protective in case of pneumonia in children. Based on this review, we planned this study to document impact of zinc supplementation on morbidity in children with pneumonia of ages 1 year to 5 years.
Aims and objectives
To evaluate impact of zinc therapy on duration of hospitalization and outcome in study cases with and without supplementation of zinc. Follow up of the children for three months to determine morbidity and mortality in a study case.
Operational definition
Pneumonia: The World Health Organization (WHO) has well-defined pneumonia especially on the base of clinical results found by visual check-up and judgment of the respiratory rate. It describes the precise cause by clinical features or chest X-ray presence. Pneumonia is categorized as;
1. Very severe pneumonia.
2. Severe pneumonia.
3. Pneumonia.
4. No pneumonia: Cough or cold.
Very severe pneumonia: Difficult breathing or cough plus at least one of the following:
• Central cyanosis.
• Severe respiratory distress.
• Failure to breastfeed or drink or vomiting all.
• Lethargy or unconsciousness and convulsions.
In addition, some or all of the other signs of pneumonia or severe pneumonia could be present such as:
• Age 2-11 months; Respiratory rate >50/minute.
• Age 1-5 years; Respiratory rate >40/minute.
• Nasal flaring.
• Fast breathing rate.
• Grunting.
• In drawing of lower chest wall.
• Chest auscultation signs of pneumonia.
• Pleural rub.
• Crackles.
• Decreased breath sounds.
• Bronchial breath sounds.
• Abnormal vocal resonance.
Severe pneumonia: Difficult breathing or cough plus at minimum one of the following signs:
• Nasal flaring.
• Lower chest wall in drawing.
• Grunting.
• Checked that are no signs of very severe pneumonia.
Others include:
• Central cyanosis
• Inability to breastfeed or drink.
• Vomiting everything.
• Convulsions, lethargy or unconsciousness.
• Severe respiratory distress.
In addition, some or all of the other signs of pneumonia or severe pneumonia may be present such as:
• Fast breathing rate
• Age 2-11 months >50/minute.
• Age 1-5 year; >40/minute.
Chest auscultation signs of pneumonia.
• Decreased breath sounds.
• Bronchial breath sounds.
• Crackles.
• Abnormal vocal resonance (decreased over a pleural effusion. increased over lobar consolidation).
• Pleural rub.
Pneumonia (non-severe): On examination we find:
• Fast breathing rate.
• Age 2-11 months respiratory rate >50/minute.
• Age 1-5 years; respiratory rate >40/minute.
Child has none of the signs of severe or very severe pneumonia.
Outcomes
Primary outcome: Duration of illness and treatment failure
Secondary outcome: Incidence of subsequent illness episodes (pneumonia and any others) in three month time with monthly follow up.
Morbidity
Duration of stay >5 days, treatment failure, repeated pneumonia, URTI and Diarrhoea, etc.
Materials and Methods
Study design
Interventional study: Randomized controlled trial.
Inclusion criteria
Any child from 1 year to 5 years ago with pneumonia consistent with operational definition admitted in Department of Paediatric Medicine, Mayo hospital/KEMU was included for the study.
Exclusion criteria
• Upper respiratory infection, common cold, otitis media, pharyngitis, acute tonsillitis.
• Foreign body aspiration, aspiration pneumonia.
• Suspected tuberculosis (fever >2 weeks duration), cough>30 days of duration, family history of contact with tuberculosis.
• Children who were on ventilatory support will be excluded from study.
Sample size
A total of 133 cases were calculated with 95% confidence level, 8.5% margin of error and taking expected percentage of pneumonia i.e., 50% in children under 5 years of age by using following formula:
Where,
Z = 95% confidence level = 1.96
P = proportion of sample = 0.50
d = margin of error = 0.085
But we took 150 children of pneumonia and divided into two groups.
Group A: Seventy five pneumonia children 1 year to 5 years supplemented with zinc therapy with dose of 20 mg/day BD for 15 days.
Group B: Seventy-Five children suffering from pneumonia aged 1 year to 5 years not supplemented with zinc (Placebo).
Setting: Department of Paediatric Medicine, Mayo Hospital, King Edward Medical University, Lahore, Pakistan, admission through outpatient department (OPD) and emergency.
Duration of study: One year - Jan 2015 to Dec 2015.
Data collection procedure: After getting consent from parents, 150 children fulfilling the inclusion criteria were registered for the study. Demographic profile for each child was recorded. Each child was evaluated for central cyanosis, convulsions, lethargy or unconsciousness, severe respiratory distress, breathing age, Inability to breastfeed or drink or vomiting everything, drawing chest wall, nasal flaring and grunting, signs of pneumonia pleural rub, crackles, decreased, breath sounds, bronchial breath sounds, abnormal vocal resonance on chest auscultation. Then children were randomly divided in two groups by using lottery method.
In group A, zinc therapy with the dose of 20 mg/day was given while in group B, placebo had started for 15 days for children. Children were given according to WHO guideline treatment and match age, sex, socioeconomic, malnutrition, well-nourished in both groups. WHO Guideline treatment, was for very severe pneumonia antibiotic therapy; Ampilillin (50) mg/kg IM every 6 hours) gentamicin (7.5 mg/kg IM once a day) for 5 days; then, if child responded well, completed treatment at home or in hospital with oral amoxicillin (15 mg/kg three times a day) plus IM gentamicin once daily for a further 5 days. Alternatively, given chloramphenicol (25 mg/kg IM or IV every 8 hours) until the child had improved. Then continued orally 4 times a day for a total course of 10 days or used ceftriaxone (80 mg/kg IM or IV once daily). Children who were not improved within 48 hours, switched to gentamicin (7.5 mg/kg IM once day) and cloxacillin (50 mg/kg/IM or IV every 6 hours), as described below for staphylococcal pneumonia. When the child improved, continued cloxacillin (or dicloxacillin) orally 4 times a day for a total course of 3 weeks. Treatment for severe pneumonia was Benzylpenicillin (50000 units/kg IM or IV every 6 hours) for at least 3 days. When the children improved, switched to oral amoxicillin (25 mg/kg, 2 times a day). The total course of treatment was 5 days. The children were improved within 48 hours, switched to cloramphenicol (25 mg/kg every 8 hours IM or IV) until the child had improved. Then continued orally for a total of 10 days. Treatment for pneumonia (non-severe) was Cotrimoxazole (4 mg/kg trimethoprim/20 mg/kg sulfamethoxazole twice a day) for 3 days or amoxicillin (25 mg/kg, 2 times for 3 days. Then children were followed-up in ward till recovery of pneumonia symptoms and discharged. Total days of stay at hospital were recorded (as per operational definition). After discharge all children were followed-up in OPD till 3 months for hospital stay of severe pneumonia. There was no missing case for follow up.
Data Analysis
The data collected from the patients of both the groups was entered in the SPSS version 13 for the purpose of analysis. For quantitative variables like age and duration of hospital stay was presented as mean and standard deviation. For qualitative variables like gender, short or long hospital stay of severe pneumonia was presented as frequency and percentage. Both groups were compared by using t-test for quantitative variables and chi-square for qualitative variables. P-value≤0.05 was taken as significant.
Results
Result shows that out of 75 children in Group-A, 26 (34.7%) children were 1 year to 2 years old, 17 (22.7%) were 2-3 years, 19 (25.3%) were 3-4 years and 13 (17.3%) children were 4-5 years old. The mean age of children in Group-A was 3.01 years. Similarly in Group-B, out of 75 children 26 (34.7%) children were 6 month to 2 years old, 17 (22.7%) were 2-3 years, 19 (25.3%) were 3-4 years and 13 (17.3%) children were 4-5 years old. The mean age of children in Group-B was 2.85 years (Table 1).
| Age | Group-A | Group-B | ||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| 6m – 2 year | 26 | 34.7 | 26 | 34.7 |
| 2-3 years | 17 | 22.7 | 17 | 22.7 |
| 3-4 years | 19 | 25.3 | 19 | 25.3 |
| 4-5 years | 13 | 17.3 | 13 | 17.3 |
| Total | 75 | 100.0 | 75 | 100.0 |
Mean Age;Group A = 3.01 ± 0.8; Group B = 2.85 ± 0.7
Table 1: Distribution of children age.
Figure 1 describes that in Group-A, 48 (64.0%) children were male while 27 (36.0%) were female. In Group-B, 48 (64.0%) children were male while 27 (36.0%) children were female.
In group A, the mean hospital stay of children was 3 ± 1 days with minimum and maximum days of 3 and 5 days. While in group B, the mean hospital stay was 7 ± 3 days with the minimum and maximum days of 3 and 10 days. The difference was significant between both groups (P<0.05).
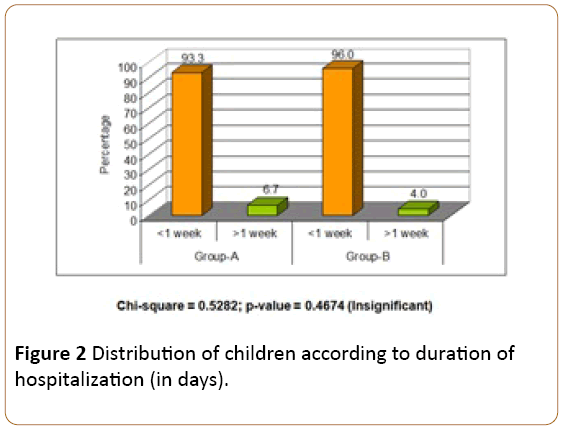
In Group-A, the duration of hospitalization of 70 (93.3%) children was less than one week and in 5 (6.7%) children duration was more than one week. In Group-B, the duration of hospitalization of 72 (96.0%) children was less than one week and 3 (4.0%) children duration was more than one week. The difference between both groups was insignificant (P>0.05) (Figure 2 and Table 2).
| Hospital stay | Study groups | |
|---|---|---|
| Group-A | Group-B | |
| Mean | 3 | 7 |
| SD | 1 | 3 |
| Minimum | 2 | ,4 |
| Maximum | 5 | 10 |
T-test = 10.9545
P-value =0.000 (Significant)
Table 2: Comparison of hospital stay in both groups.
Discussion
Zinc plays vital role in the repair of host defenses against infection [16]. The zinc deficiency is public problem worldwide [17,18]. The therapeutic benefit of oral zinc is reported to lower risk of acute respiratory infections and pneumonia in children. The zinc supplementation results in significant reduction in the duration of hospital stay in children [19]. Keeping these considerations, the present study was conducted out to find out the effect of co-administration of zinc with standard antimicrobial therapy in a double blind randomized controlled clinical trial in 150 children with pneumonia.
In our study, with zinc therapy, 70 (93.3%) children were discharged from hospital within one week while with placebo group, 72 (96.0%) children were discharged from hospital within one week. There were more children in placebo group who were discharged from hospital within 1 week although the difference between both groups was insignificant (P>0.05).
Results of present study are comparable with the trial by Brook et al. [20,21] in Bangladesh. Zinc supplementation given with empiric antimicrobial therapy significantly shortened the duration of hospital stay for young children with pneumonia [22]. Iqbal et al. also reported similar results from Pakistani children [23], Qasemzadeh et al. from Iran reported significant decrease was found in the duration of hospitalization and recovery from severe pneumonia symptoms in zinc receiving children [24]. In contrast two trails from Nepal demonstrated that adjuvant zinc neither reduced the risk of treatment nor hasten the recovery from non-severe or severe pneumonia in Nepalese children in the age group of 2-35 months of age [20]. Mahalanabis et al. at Kolkata, India done research that shows short duration of recovery in pneumonia then placebo control groups. [19,20]. The difference trials could be well explained if pre and post treatment plasma zinc levels estimation would have done. Present study has the some limitation that we did not measure serum levels zinc [16].
In our study, with zinc therapy, 73 (97.3%) children were discharged alive from hospital and remained alive during the course of study (3 months) while with placebo, 55 (73.3%) children were discharged alive from hospital and remained alive during the course of study (3 months). The difference between both groups was highly significant (P<0.05) But Wadhwa et al. found that zinc therapy is given or placebo is given, the death rate will be equal i.e., 1.5% with either method [25].
Conclusion
The results of this study have shown that addition of zinc as adjunct to the conventional therapy for management of pneumonia is more effective than conventional treatment without zinc. This is single center study. Multi-center studies are needed on a large scale to further prove the benefit of zinc as adjunct to conventional therapy for management of severe pneumonia in children.
References
- UNICEF (2006) Pneumonia the forgotten killer of children.
- Khan TA, Madni SA, Zaidi AK (2004) Acute respiratory infection in Pakistan:Have we made any progress? JColl physicians Surg Pak14:440-448.
- Mullholland K (2003) Global burden of acute respiratory infections in children:Implications for interventions. PediatrPulmonal36: 469-474.
- Hambidge KM (1981) Zinc deficiency in man: Its origin and effects. Philos Trans R SocLond B BiolSci294:129-144.
- Feigin RD (2003) Textbook of pediatric infectious diseases.(5th edn). Philadelphia: W. B. Saunders p: 299.
- Trunong-tran AQ, Ruffin RE, Foster PS, Koskinen AM, Coyle P, et al. (2002) Altered zinc homeostasis and caspase-3 activity in murine allergic airway inflammation. AmJ Respir CellMolBiol 27: 286-296.
- Theodare SC, Prober CG (2008) Pneumonia. Nelson text book of paediatrics, (18th edn). Philadelphia p:1795-1799.
- Cho YH, Lee SJ, Lee JY (2002) Antibacterial effects of intraprostatic zinc injection in a rat model of bacterial prostatitis. Int J Antimicrob agent19:576-582.
- BlackRE, Morris SS,BryceJ(2003) Where and why are 10 million children dying every year? Lancet36:2226-2234.
- Müller O, Becher H, Van Zweeden AB, Ye Y, Diallo DA, et al. (2001) Effect of zinc supplementation on malaria and other causes of morbidity in West African children: Arandomized double blind placebo controlled trial. BMJ 322:1507-1570.
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE (2005) The WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children? Lancet365:1147–1152.
- IolasconG, GimiglianoR, BiancoM, De Sire A, Moretti A, et al.(2016) Are dietary supplements and nutraceuticals effective for musculoskeletal health and cognitive function? A scoping review. J Nutr Health Aging p: 1-12.
- McBurneyMI,Hartunian-Sowa S, MatusheskiNV(2017) Implications of US Nutrition Facts: Label changes on micronutrient density of fortified foods and supplements. J Nutr.
- Wakaskar RR, Bathena SP, Tallapaka SB, Ambardekar VV (2015) Peripherally cross-linking the shell of core-shell polymer micelles decreases premature release of physically loaded combretastatin A-4 in whole blood and increases its mean residence time and subsequent potency against primary murine breast tumors after IV administration. Pharm Res 32:1028-1044.
- Shankar AH, Prasad AS (1998) Zinc and immune function: the biological basis of altered resistance to infection. Am J clinNutr68: 447s-463s.
- Mahalanabis D, Lahiri M, Paul D, Gupta S, Gupta A, et al. (2004) Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J ClinNutr79:430–436.
- AmbardekarVV, WakaskarRR, SharmaB, BowmanJ, Vayaboury W, et al. (2013) The efficacy of nuclease-resistant Chol-siRNA in primary breast tumors following complexation with PLL-PEG (5K). Biomaterials 34: 4839-4848.
- Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS (2003) How many child deaths can we prevent this year? Lancet362:65–71.
- Bose A, Coles CL, GunavathiL(2006) Efficacy of zinc in the treatment of severe pneumonia in hospitalized children <2 years old. Am J Clin Nutr,83:1089–1096.
- Valentiner-Branth P (2010)A randomized controlled trial of the effect of zinc as adjuvant therapy in children 2-35 monthsof age with severe or non-severe pneumonia in Bhaktapur, Nepal. Am J ClinNutr91:1667-1674.
- Brooks WA, Yunus M, Santosham M (2004) Zinc for severe pneumonia in very young children: Adouble-blind placebo-controlled trial. Lancet363:1683–1688.
- Brooks WA, Santosham M, Naheed A (2005) Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: Arandomised controlled trial. Lancet366:999–1004.
- Iqbal I, Mahmood S, Tariq A (2010) Effect of oral zinc supplementation on duration of illness and mortality in children on conventional treatment for pneumonia. Nishtar Med J 2: 51-55.
- QasemzadehMJ, Fathi M, TashvighiM(2014) The effect of adjuvant zinc therapy on recovery from pneumonia in hospitalized children: A double-blind randomized controlled trial.Scientifica.
- Wadhwa N, Chandran A, Aneja S, Lodha R, Kabra SK, et al. (2013) Efficacy of zinc given as an adjunct in the treatment of severe and very severe pneumonia in hospitalized children 2–24 monthsof age: A randomized, double-blind, placebo-controlled trial. Am J ClinNutr97:1387-1394.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences